医薬品・医療機器 GxP CSV要件に応える
コンサルティングサービス
NEWS
FDA (FAERS) : ICH E2B 個別有害事象電子報告に関連する2種類のレギュレーション・ドキュメントを Jan-2023 付で改訂FDA: Updated "FDA Adverse Event Reporting System (FAERS) Electronic Submissions" on 31-Jan-2023
HiroPharmaConsulting Newsletter Vol.5 No.4をアップいたしました。
今回は、久しぶりに更新されました、FDA FAERS ESM Web Pageのご案内です。
1) 変更点は以下の2点です。
-
- FDA E2B(R3) Core and Regional Data Element v1.0 on Jan-2023 :「一部 Data Element仕様の変更」
- FDA ICSR XML Instances on Jan-2023:「XMLインスタンスの更新」
2) なお、ICH E2B(R3) xml Formatでの電子報告はまだ開始されていません。引き続き以下のNoteが継続されています。
Please note, FDA is not currently accepting the submission of postmarketing ICSRs in the E2B(R3) format.
3) また、直接 FDA FAERS Teamへ『ICH E2B(R3) でのFDA報告開始時期』の問合せを2023/01/27 (金)行いましたが、以下のような返事が届いております。
Hi,
We do not have any updates. Please follow the FAERS site as all updates will be posted there.
Thanks,
————————————————————————————————————————————–
FDA: Updated “FDA Adverse Event Reporting System (FAERS) Electronic Submissions” on 31-Jan-2023
——————————————————————————————————–
1. FDA E2B(R3) Core and Regional Data Element v1.0 on Jan-2023
https://www.fda.gov/media/157982/download
“FDA-E2B-R3-Core-and-Regional-Data-Elements-and-Business-Rules-Version-1-3.xlsx”
[Jan 2023: v1.3での変更点]
|
1.3 |
Jan-2023 |
• All data elements specific to vaccine reporting are hidden and must not be used of non-vaccine reporting |
————————————————
2. FDA ICSR XML Instances on Jan-2023
https://www.fda.gov/media/157983/download
“FDA-ICSR-XML-Instances_0.zip”
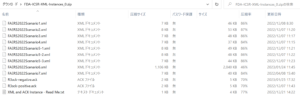
FDA-ICSR-XML-Instances_0.zip
————————————————-
[FDA FAERS Electronic Submissions Web Page]
FDA Adverse Event Reporting System (FAERS) Electronic Submissions
· Content current as of:
01/30/2023
Updates for Electronic Submission of Individual Case Safety Reports (ICSRs) to FAERS
FDA recently updated the following guidances for industry to incorporate technical updates: E2B (R3) Electronic Transmission of Individual Case Safety Reports Implementation Guide – Data Elements and Message Specification and Appendix to the Implementation Guide — Backwards and Forwards Compatibility.
Premarketing Safety Reporting
In preparation for the electronic transmission of premarketing safety reports in the International Council for Harmonisation (ICH) E2B(R3) format, FDA has posted the following documents regarding the electronic submission of ICSRs for certain investigational new drug application (IND) safety reports for drug and biological products and IND-exempt bioavailability/bioequivalence (BA/BE) safety reports to FAERS. These documents are posted to help sponsors prepare their systems for electronic submission of IND safety reports in the E2B(R3) format.
- Providing Regulatory Submissions in Electronic Format: IND Safety Reports – Draft Guidance for Industry (October 2019)
- Electronic Submission of IND Safety Reports – Technical Conformance Guide (April 2022)
- Technical Specifications Document – FDA Regional Implementation Guide for E2B(R3) Electronic Transmission of Individual Case Safety Reports for Drug and Biological Products (August 2022)
- Electronic Submission of Expedited Safety Reports From IND-Exempt BA/BE Studies – Draft Guidance for Industry (August 2022)
- FDA E2B(R3) Core and Regional Data Elements and Business Rules (Excel file January 2023)
- FDA ICSR XML Instances (zip file January 2023)
Please note, FDA is not currently accepting the submission of premarket ICSRs in the E2B(R3) format. Please continue to submit IND safety reports using eCTD format and IND-exempt BA/BE safety reports on Form FDA 3500A. FDA will update this web page when final guidance for IND safety reporting is published, and when FDA will accept IND and IND-exempt BA/BE safety reports in E2B(R3) format on a voluntary basis. FDA will also update this web page to communicate when submission of safety reports in E2B(R3) format is required for certain INDs after the period of voluntary submission.
Postmarketing Safety Reporting
In preparation for the receipt of postmarketing safety reports in the E2B(R3) format, FDA has posted the following documents regarding the electronic submission of safety reports for drug and biological products to FAERS. These documents are posted to help prepare systems for electronic submissions of postmarketing safety reports.
- Technical Specifications Document – FDA Regional Implementation Guide for E2B(R3) Electronic Transmission of Individual Case Safety Reports for Drug and Biological Products (August 2022)
- FDA E2B(R3) Core and Regional Data Elements and Business Rules (Excel file January 2023)
- FDA E2B(R3) Forward Compatible Rules (Excel file April 2022)
- FDA ICSR XML Instances (zip file January 2023)
- Providing Submissions in Electronic Format – Postmarketing Safety Reports: Guidance for Industry (April 2022)
Please note, FDA is not currently accepting the submission of postmarketing ICSRs in the E2B(R3) format. FDA will update this web page when postmarketing ICSRs will be accepted in the E2B(R3) format. In the meantime, please continue to submit postmarketing ICSRs in the E2B(R2) format.
For questions related to this update, please contact the FAERS electronic submission coordinator at faersesub@fda.hhs.gov.
